Calibration in pharmaceuticals is one of the most fundamental activities in the industry. Every measurement we record—temperature, pressure, weight, pH, time, speed, humidity—directly affects product quality, patient safety, and regulatory compliance.
Everyone working in the pharmaceutical industry must understand why calibration is required, how it is controlled, and what regulators actually expect.
This blog explains calibration in a simple way, so that any pharma professional can clearly understand the complete calibration lifecycle.
What is Calibration?
Calibration is the process of comparing the readings of an instrument with a known, traceable reference standard to determine whether the instrument is measuring accurately within acceptable limits.
In simple words, calibration answers one basic question:
“Is my instrument telling the truth?”
If the instrument’s readings match the reference standard within predefined limits, the instrument is considered calibrated. If not, it requires adjustment, repair, or replacement.
Why Calibration Is Critical
In pharmaceuticals, decisions are based entirely on measurements. Even a small error can lead to:
- Incorrect batch release decisions
- Product quality failures
- Stability failures
- Regulatory observations (FDA, WHO, EU GMP, etc.)
- Risk to patient safety
For example:
- An incorrect balance reading can affect dosage strength.
- A faulty temperature sensor can invalidate a sterilization cycle.
- A wrong pH reading can cause product instability.
This is why regulators treat calibration as a GMP-critical activity, not a technical formality.
What Instruments Require Calibration in Pharma?
In a pharmaceutical facility, any instrument that measures, monitors, or controls a critical parameter must be calibrated.
This typically includes:
- Weighing balances
- Temperature sensors, probes, and recorders
- Pressure gauges and transmitters
- pH meters
- Conductivity meters
- Timers and stopwatches
- Flow meters
- RPM indicators
- Hygrometers (humidity sensors)
- Autoclaves, ovens, incubators, and chambers (via their sensors)
A simple rule to remember:
If an instrument influences product quality, safety, or compliance — it must be calibrated.
How Calibration Is Performed
Calibration always involves three key elements:
- Instrument Under Calibration (IUC)
The device whose accuracy needs to be verified. - Reference Standard
A highly accurate, certified instrument with known values. - Acceptance Criteria
Predefined limits that decide pass or fail.
The instrument’s reading is compared against the reference standard at multiple points. Any deviation is evaluated against acceptance criteria.
Steps in a Calibration Activity
Although procedures vary, the basic steps remain the same across pharma companies:
- Identification of the instrument: Instrument ID, location, and calibration status are verified.
- Verification of reference standard: Ensure the standard used is valid, calibrated, and traceable.
- Execution of calibration checks: Readings are taken at defined points across the operating range.
- Recording of results: Observed values, standard values, and deviations are documented.
- Assessment against acceptance criteria: Instrument is declared pass or fail.
- Labeling and status update: Calibration status label is affixed and records are updated.
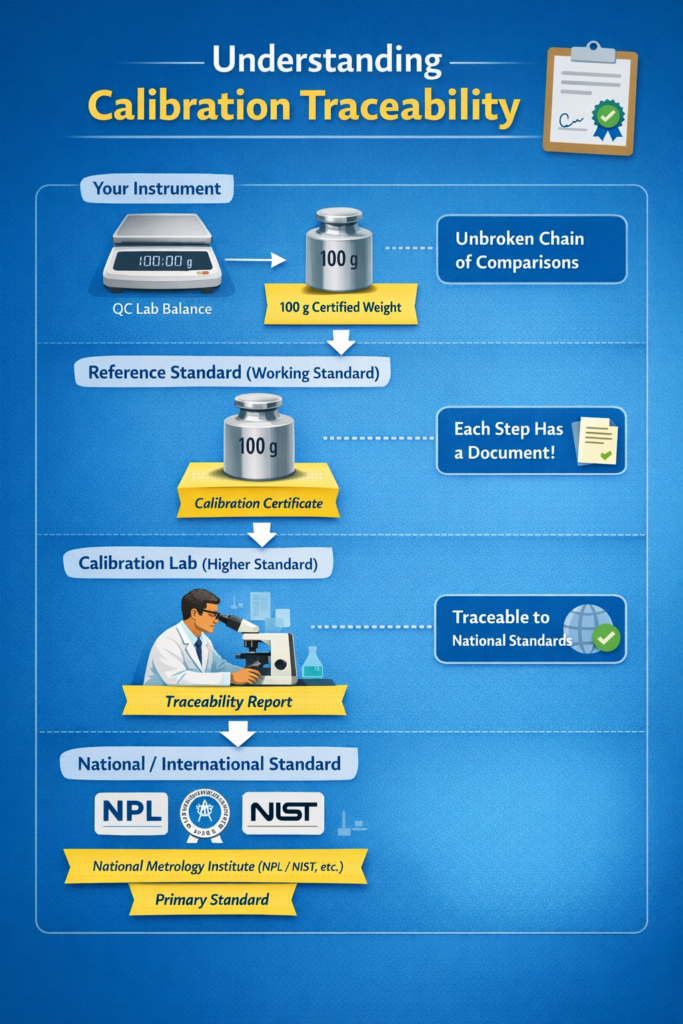
What Is Calibration Traceability?
Calibration is meaningless without traceability.
Traceability means that the reference standard used for calibration is linked through an unbroken chain of comparisons to a national or international standard (such as NPL, NABL, NIST).
In audits, regulators often ask:
“Show me the traceability of this calibration.”
If traceability is missing, calibration is considered invalid, even if readings look correct.
Acceptance Criteria
Acceptance criteria define how much deviation is acceptable.
They are usually based on:
- Instrument criticality
- Process requirement
- Manufacturer’s specification
- Regulatory expectations
For example:
- ±0.1°C for temperature sensors
- ±0.01 g for analytical balances
These limits must be defined in procedures, not decided during calibration.
Frequency
Calibration frequency is the time interval between two consecutive calibrations of an instrument.
Common frequencies include:
- Daily
- Weekly
- Monthly
- Quarterly
- Half-yearly
- Yearly
Calibration Frequency is determined based on:
- Instrument criticality
- Stability of the instrument
- Historical calibration data
- Manufacturer recommendation
- Regulatory expectations
Critical instruments used in sterile manufacturing or QC labs usually have shorter calibration intervals.
Changing of calibration frequency is possible but only through a controlled, documented approach.
Frequency changes must be supported by:
- Historical trend data
- Risk assessment
- QA approval
Regulators expect scientific justification, not convenience-based decisions.
Calibration Schedule: Controlling the Entire Program
A calibration schedule is a planned, documented calendar that lists:
- Instrument ID
- Location
- Calibration frequency
- Due dates
- Responsible department
This ensures every instrument is calibrated without missing.
A well-maintained schedule helps to:
- Prevent use of overdue instruments
- Demonstrate GMP control during audits
- Support effective workload planning
- Avoid last-minute compliance issues
Most pharma companies maintain calibration schedules electronically or in controlled registers.
Internal vs External Calibration
Internal Calibration
Performed using in-house standards by trained staff. Suitable for:
- Routine instruments
- Non-complex measurements
External Calibration
Performed by certified agencies (e.g., NABL-accredited). Required for:
- Critical instruments
- Reference standards
- High-accuracy measurements
Both approaches must meet GMP and traceability requirements.
Common Calibration Mistakes in Pharma
Some frequent issues seen during audits:
- Using expired reference standards
- Missing traceability certificates
- Overdue calibration
- Inadequate impact assessment
- Poorly defined acceptance criteria
- Missing calibration labels
Handling Out-of-Calibration Instruments
If an instrument fails calibration:
- It must be labeled “Out of Calibration”
- Impact assessment must be performed
- A deviation may be required
- Product quality impact must be evaluated
Ignoring this step is a serious GMP violation.
Validating Depyrogenation Process: Meeting Ph. Eur. 5.1.12 Guidelines




