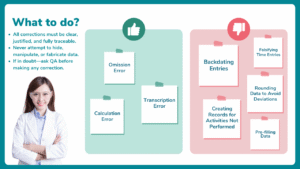
Documentation errors in pharma are the most common reasons for audit observations. These errors may occur even in well-established facilities. These errors rarely occur due to a lack of technical knowledge. Instead, they originate from day-to-day operational pressures, assumptions, and workload. Unfortunately, auditors consider documentation as factual evidence. No matter how truthful the operator is, any gap in documentation can quickly escalate into a compliance concern.
On the shop floor, activities move fast. Operators and analysts focus on completing tasks safely and meeting timelines. However, when documentation does not accurately reflect what actually happened, when it happened, and who performed it, auditors begin to question not just the record, but the entire control system behind it. In pharma, what is not documented is considered not done, regardless of how well the activity was executed.
Let’s look at some real audit scenarios observed on the shop floor, not theoretical GMP examples. Each case highlights how simple documentation gaps, often considered minor by operators, have resulted in audit observations.
The objective is not to assign blame, but to help teams understand how auditors think and why documentation discipline is critical for regulatory compliance.

1. Logbook Entry Made After the Activity Was Performed
In one case, an analyst forgot to make his entry in the cleanroom entry logbook before entering the manufacturing area. He entered the area, performed his assigned activity, and exited the cleanroom.
Later, when he realized that he had missed the entry, he went back to the logbook. By that time, two other operators had already made their entries. The analyst then wrote his entry below theirs, even though his actual activity had occurred earlier.
During the audit, the inspector compared:
- Logbook entry sequence
- Time of activity
- Batch manufacturing records
The sequence did not match the actual activity timeline. The auditor concluded that the logbook was not contemporaneous and did not reflect actual events, and this was recorded as a documentation deficiency.
This situation is critical because entry logbooks are legal documents used to reconstruct events. When entries are not made in real time, the integrity of the entire activity becomes questionable.
If an entry is missed, it must be immediately escalated as a documentation error or deviation, rather than being silently corrected later.
2. Activity Performed but Never Documented Due to Absence
In this scenario, an operator performed an activity but postponed documentation, planning to complete it the next day. Unfortunately, he became ill the following day and was unable to attend work for a week.
When he returned to work, he forgot about it, and the activity was closed. There was no opportunity to document it retrospectively either. Eventually, during an audit, the missing documentation was identified.
The auditor observed that the activity had no contemporaneous record and concluded that it was either not performed or not properly controlled, as per GMP expectations.
Auditors do not consider personal situations such as illness or leave. They need evidence that the activity was performed as required.
Never leave documentation for later. If it is not written today, it may never be written.
3. Pre-Documentation of an Activity That Was Never Performed
After making all arrangements, the operator anticipated performing an activity and documented it in advance, expecting to start immediately. However, due to an HVAC issue, clearance was not given, and the activity was never initiated.
During a routine review, QA noticed that the activity was documented but lacked corresponding execution, approval, or clearance.
This was flagged as pre-documentation, which is considered a serious GMP violation because it creates false records. Even though the operator’s intention was not wrong, the documentation falsely indicated that the activity had occurred.
From an audit standpoint, this is a data integrity issue, not a procedural error.
Documentation must follow the activity—not precede it.
4. Calibration Due Date Entered from Memory Instead of Reference
An operator was responsible for routinely recording calibration due dates for an instrument. Since the due date had remained unchanged for several months, he entered it from memory without verifying the calibration label or certificate.
However, an interim calibration had been performed as part of a CAPA, which changed the due date. The operator was unaware of this change and documented the old due date.
During the audit, the inspector cross-checked:
- Calibration certificate
- CAPA records
- Equipment logbook
The mismatch was immediately identified, and the auditor concluded that calibration records were not verified against controlled documents.
This raised concerns about equipment status awareness and reliance on memory instead of data.
Memory is not a GMP-approved reference source.
5. Activity Performed and Documented but Not Signed
In another instance, an operator performed an important GMP activity and documented all required details accurately. However, he forgot to sign the record at the end of the activity.
During the audit, the inspector reviewed the document and asked:
“Who performed this activity?”
Although everyone in the department knew the operator, there was no documented proof. The auditor noted that without a signature, the record lacks ownership and accountability.
From an audit perspective, an unsigned document is considered incomplete and unofficial. Even if the activity was technically correct, the absence of a signature invalidates the record.
Documentation without a signature is treated the same as no documentation at all.
Closing Message to the Team
All these examples have one common lesson:
In GMP, what you do is important—but what you document is what gets audited.
Documentation must be:
- Contemporaneous
- Accurate
- Complete
- Traceable
When in doubt, stop the activity and seek guidance.
A documented mistake can be corrected—but an undocumented activity cannot be defended.

ALCOA Principles in Pharmaceuticals
10 Habits That Prevent Errors in Documentation in Pharma
Pharmaceutical Documentation: Documented But Not Performed
What Kind of Entry Errors Are Acceptable in Pharma Documentation?
Common Documentation Errors in the Pharmaceutical Industry