In the pharmaceutical industry, every documented entry is a legal record of what has been done. Regulatory authorities expect these records to be complete, accurate, and contemporaneous. Even small errors can cause major compliance issues, create data integrity concerns, and impact product safety. Let’s explore the common documentation errors in the pharmaceutical industry.
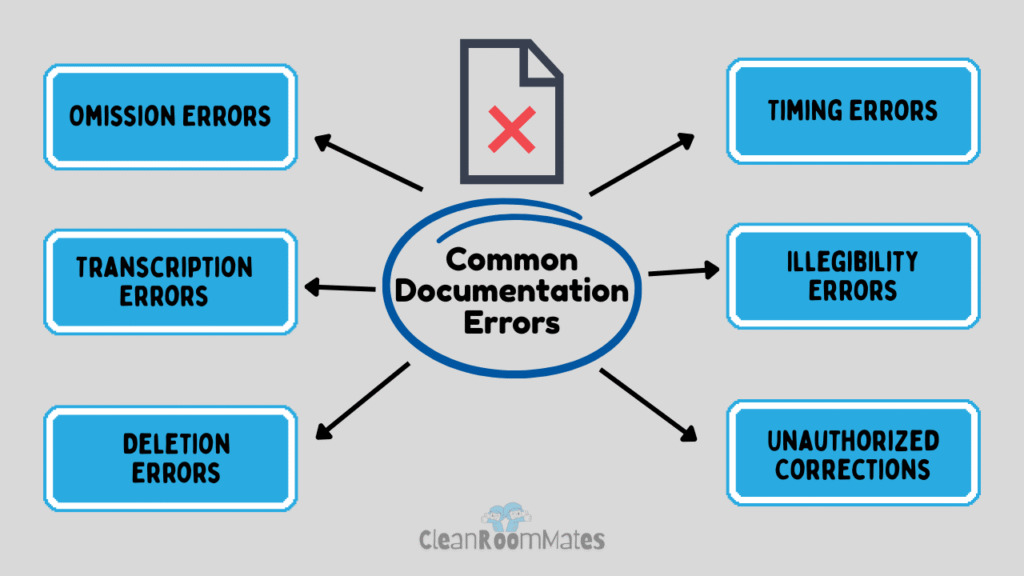
1. Omission Errors
Omission errors occur when required information is left blank or completely missing from a record. These errors often result from oversight, time pressure, or insufficient understanding of documentation requirements.
Example: A laboratory analyst forgets to record the cleaning activity details in the equipment logbook. When the oversight was noticed, the other operators had already made multiple entries in the logbook. This makes it difficult to reconstruct the exact cleaning timeline.
Impact:
- Missing information breaks traceability of who performed an activity and when.
- It can lead to questions about whether the activity was performed at all.
- Regulatory bodies may classify omissions as critical data integrity failures.
- Investigations and deviations must be raised to reconcile and explain missing data.
2. Transcription Errors
Transcription errors happen when information is copied incorrectly from one record or instrument to another. These often occur during manual data transfer.
Example: An analyst transcribes the temperature from an incubator display as 25.0 °C instead of the correct 35.0 °C into the logbook. Another example is a QC chemist transcribes the batch number as B12346 when the correct batch number was B12364.
Impact:
- Leads to incorrect data records, which may invalidate test results.
- Compromises product quality and regulatory compliance.
- May require retesting, revalidation, and investigation.
- Reduces confidence in data integrity across the system.
3. Deletion Errors
Deletion errors involve removing or erasing information without following proper correction procedures.
Example: An operator crosses out a recorded cleaning time completely and rewrites a new time, but fails to provide any reason, initials, or date.
Impact
- Deletion of data violates ALCOA+ principles, particularly “Original”.
- Can be seen as intentional falsification.
- Regulatory agencies treat such errors as critical non-compliance.
4. Timing Errors
Timing errors occur when the date or time entered does not match reality. It may happen either by mistake or intentionally.
Example: An operator records the cleaning completion time as 2:00 PM, but he had finished the cleaning at 4:00 PM.
Impact
- Creates discrepancies between documented and actual timelines.
- Raises suspicion of pre-signing or backdating records.
- Can lead to data integrity investigations.
5. Illegibility Errors
Illegibility errors occur when entries are difficult or impossible to read because of poor handwriting, overwriting, or using inappropriate writing materials.
Example: A technician’s handwriting is so unclear that “1.29” appears to be “7.29”.
Impact
- Makes the verification and review process difficult.
- Potentially invalidates records if reviewers or inspectors cannot read them.
- Causes delays in batch release and document approval.
6. Unauthorized Corrections
When an entry error is corrected without following proper GDP procedures, such as failing to sign, date, or explain the correction.
Example: Applying correction fluid (white-out) to cover a wrong entry and writing the new value over it. Crossing out incorrect data and entering new data without initials, date, or reason for the change.
Impact
- Violates GDP expectations of transparency and traceability.
- Auditors consider this an attempt to hide or manipulate data.
- Leads to regulatory findings and potentially invalid records.
All of these types of documentation errors have serious implications. They compromise the principles of ALCOA+—Attributable, Legible, Contemporaneous, Original, Accurate, Complete, Consistent, Enduring, and Available—and damage the credibility of records. It requires thorough training, a culture of accountability, clear procedures, and consistent review to prevent these errors. In the pharmaceutical industry, every piece of documentation must be complete, accurate, and defensible to protect patients and maintain regulatory compliance.
You may also be interested in reading the following.
Top 10 Reasons Behind Human Errors in the Pharmaceutical Industry
Pharmaceutical Audits: How to Manage Stress During Audits?