ALCOA principles are the foundation of data integrity in the pharmaceutical industry. Every test result, record, and decision in a GMP environment must comply with ALCOA principles. Whether it is a microbiology test, batch manufacturing record, or environmental monitoring data, ALCOA defines how data should be generated, recorded, and maintained throughout its lifecycle.
What Is Data Integrity?
Data integrity refers to the completeness, consistency, and accuracy of data from the moment it is captured until it is archived. Regulatory authorities define data integrity as data that is reliable, traceable, and available for review, inspection, or audit at any time. Ensuring data integrity ultimately supports product quality and safeguards patient health.
Breaking Down ALCOA Principles
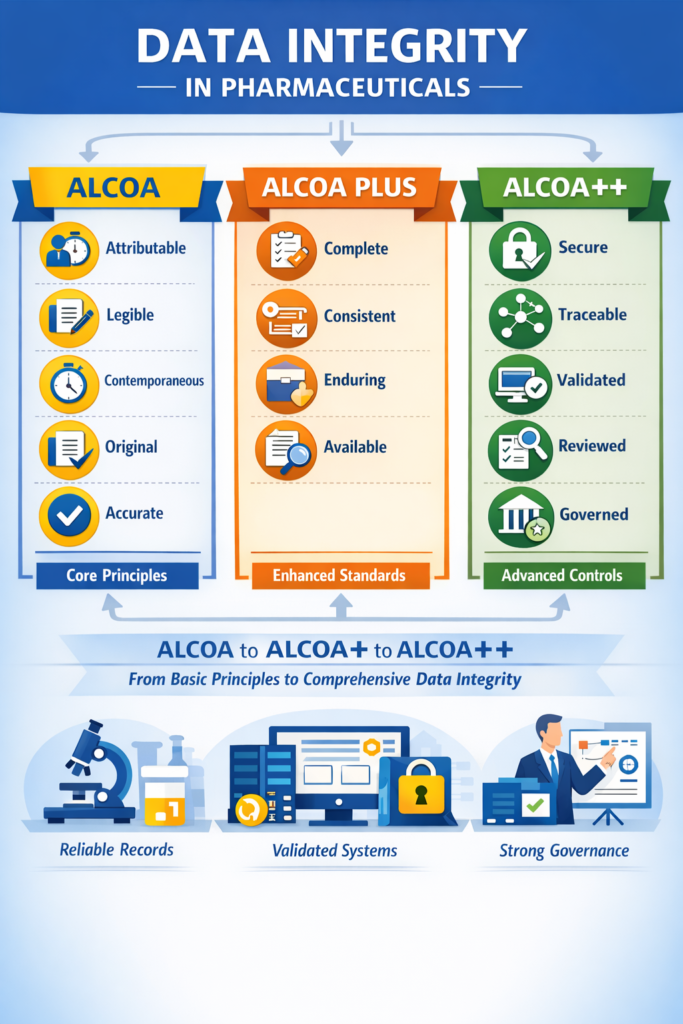
ALCOA is an acronym that outlines five foundational attributes of trustworthy data:
Attributable
Every piece of data should clearly show who recorded it and when it was done. If corrections are made, the individual making the change must also be identified.
Legible
Data must be easy to read and understand throughout its retention period. Whether it’s written on paper or stored electronically, readability is crucial.
Contemporaneous
Information should be recorded at the time the activity occurs — not later. This ensures the data reflects real-time conditions and actions.
Original
The first recorded version of data, or a certified true copy, must be preserved. This prevents loss of context and ensures traceability.
Accurate
Data must correctly reflect the results or observations without errors. If corrections are needed, they should be properly documented with justification.
By applying these five attributes, organizations ensure that data is credible, traceable, and dependable.
Evolving to ALCOA Plus (ALCOA+)
As technology and data environments became more complex — especially with electronic systems — regulators expanded ALCOA into ALCOA Plus by adding four more attributes that further reinforce data integrity.
Complete
Every piece of data must be present — including raw data, repeat results, and information about corrections and deletions. Nothing should be omitted.
Consistent
Data entries should follow a logical, chronological order. Timestamps, versioning, and audit trails help maintain consistency over time.
Enduring
Data should remain intact and readable for the entirety of its retention period, whether on paper or in electronic form.
Available
Authorized personnel must be able to access data whenever required for review or inspection. Data should not be lost, locked away, or inaccessible.
Together, these nine attributes make ALCOA Plus a stronger, more comprehensive framework for data integrity.
What is ALCOA++?
As pharmaceutical data systems became more advanced and highly digitalized, regulators realized that even ALCOA Plus needed further strengthening. This led to the informal but widely accepted concept of ALCOA++.
ALCOA++ builds on ALCOA and ALCOA Plus by adding a deeper focus on data governance, system controls, and quality culture. While ALCOA and ALCOA Plus define what good data looks like, ALCOA++ emphasizes how organizations ensure data remains trustworthy at all times.
ALCOA++ is not an official regulatory acronym, but it is increasingly referenced in inspections, audits, and data integrity guidance documents.
Additional Elements of ALCOA++
ALCOA++ expands the framework by reinforcing controls around data handling, review, and oversight. The commonly accepted additions include:
Secure
Data must be protected from unauthorized access, modification, or deletion.
- Role-based access control in computerized systems
- Strong password policies and login restrictions
- Prevention of shared user IDs
✔️ If data can be altered without traceability, it fails data integrity expectations.
Traceable
Every action performed on data should be traceable throughout its lifecycle.
- Clear audit trails showing creation, modification, and deletion
- Metadata review during investigations and audits
- Linkage between raw data, reports, and decisions
✔️ Traceability ensures accountability and supports regulatory confidence.
Validated
Systems that generate or store data must be validated for their intended use.
- Validation of computerized systems (Example: LIMS)
- Periodic review and revalidation after system changes
- Controlled spreadsheets and tools
✔️ Unvalidated systems undermine the credibility of even accurate data.
Reviewed
Data must be routinely reviewed by competent and authorized personnel.
- Second-person review of laboratory data
- Audit trail review as part of batch release
- QA oversight of critical GMP records
✔️ Data that is not reviewed is considered uncontrolled.
Governed
Organizations must have strong data governance policies and procedures.
Data integrity SOPs
- Clear ownership of data and systems
- Defined escalation and investigation processes
✔️ Governance ensures consistency across departments and sites.
Governed
Organizations must have strong data governance policies and procedures.
Pharmaceutical relevance:
- Data integrity SOPs
- Clear ownership of data and systems
- Defined escalation and investigation processes
✔️ Governance ensures consistency across departments and sites.
How to Implement ALCOA Principles
Improve Documentation Practices
- Record data immediately
- Use permanent ink or validated electronic systems
- Document corrections with rationale, date, and initials
🖥 Upgrade Electronic Systems
- Validate computer systems according to regulatory standards
- Enable audit trails for every data entry and modification
- Restrict access with secure credentials
📘 Train Employees
- Regularly educate staff on data integrity and ALCOA principles
- Encourage a culture of accountability and transparency
🧪 Conduct Internal Reviews
- Perform routine audits of records and data processes
- Review audit trails and metadata for compliance
These steps help organizations consistently apply ALCOA and ALCOA Plus in all GxP activities from manufacturing and laboratory testing to quality control and product release.
ALCOA++, when combined with ALCOA and ALCOA Plus, represents a mature and proactive approach to data integrity. It shows how pharmaceutical companies must manage data in an era of automation, electronic records, and global regulatory scrutiny.
Organizations that adopt ALCOA++ principles not only reduce compliance risks but also build long-term trust in their data, decisions, and products.
Common Documentation Errors in the Pharmaceutical Industry
What Kind of Entry Errors Are Acceptable in Pharma Documentation?
Pharmaceutical Documentation: Documented But Not Performed




